
-
 Liverpool tame Wolves to reach FA Cup quarter-finals
Liverpool tame Wolves to reach FA Cup quarter-finals
-
Kane-less Bayern brush aside Gladbach to continue title march

-
 Berger extends lead midway through Arnold Palmer Invitational
Berger extends lead midway through Arnold Palmer Invitational
-
Paralympics open with Russian athletes booed in ceremony

-
 Cuba 'next' on agenda, after Iran: Trump
Cuba 'next' on agenda, after Iran: Trump
-
Zverev leads way into Indian Wells third round

-
 NASA defense test kicked asteroid off course -- and changed its orbit around the sun
NASA defense test kicked asteroid off course -- and changed its orbit around the sun
-
Anthropic vows court fight in Pentagon row

-
 'Harder path': Obama attacks Trump at Jesse Jackson memorial
'Harder path': Obama attacks Trump at Jesse Jackson memorial
-
Amber Glenn says will not visit White House to celebrate Olympic gold

-
 Russian athletes booed as they parade under own flag at Paralympics opening
Russian athletes booed as they parade under own flag at Paralympics opening
-
Trump to attend return of six US troops killed in Iran war

-
 Tom Brady flag football event moved from Saudi to Los Angeles: reports
Tom Brady flag football event moved from Saudi to Los Angeles: reports
-
UN chief slams 'unlawful attacks', says Mideast could spiral out of control

-
 Middle East war a new shock for financial markets
Middle East war a new shock for financial markets
-
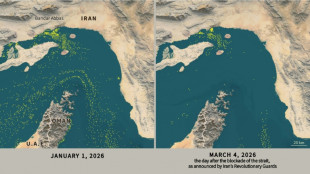
Only nine commercial ships detected crossing the Hormuz Strait since Monday
-
 Mexico unveils 100,000-strong security deployment for World Cup
Mexico unveils 100,000-strong security deployment for World Cup
-
Trump's Iran war violates international law, experts say

-
 Swiss eyeing fewer F-35 fighters, reshaping defence set-up
Swiss eyeing fewer F-35 fighters, reshaping defence set-up
-
UK police question three women in Al-Fayed probe

-
 Oil prices surge as Mideast war rages, stocks fall on US jobs
Oil prices surge as Mideast war rages, stocks fall on US jobs
-
Dupont says France must forget Six Nations title talk against Scotland

-
 Voices from Iran: protests, fear and scarcity
Voices from Iran: protests, fear and scarcity
-
Champions League ambitions encourage Barca gamble in Bilbao

-
 This is how Ukraine has countered Russia's Iran-designed drones
This is how Ukraine has countered Russia's Iran-designed drones
-
Dybala out for six weeks as Roma battle for top-four spot

-
 Sleepless Iranians count cost of war as damage mounts
Sleepless Iranians count cost of war as damage mounts
-
Itoje tells faltering England to 'take the game to Italy' in Six Nations

-
 Leading satellite firm to hold back Gulf state images
Leading satellite firm to hold back Gulf state images
-
Tuipulotu urges Scotland to stay in Six Nations title hunt against France

-
 Trump says only Iran's 'unconditional surrender' can end war
Trump says only Iran's 'unconditional surrender' can end war
-
US releases Epstein files with uncorroborated Trump allegations

-
 Securing shipping lane from Mideast war 'challenging', say experts
Securing shipping lane from Mideast war 'challenging', say experts
-
Italy have to start beating the best, says captain Lamaro

-
 India's Bumrah only 'human' says Phillips ahead of T20 World Cup final
India's Bumrah only 'human' says Phillips ahead of T20 World Cup final
-
Oil prices climb as Mideast war rages, stocks fall on US jobs

-
 US retail sales decline as consumer pullback deepens
US retail sales decline as consumer pullback deepens
-
War in Middle East raises stagflation fears in Europe and beyond

-
 UN demands swift probe into Israeli strikes on Lebanon
UN demands swift probe into Israeli strikes on Lebanon
-
Chelsea happy to rotate goalkeepers, says Rosenior

-
 Soaring gas prices spark renewed debate about European electricity
Soaring gas prices spark renewed debate about European electricity
-
Elite pilots and US support drive Israel's air power

-
 Germany's Axel Springer swoops for British newspaper The Telegraph
Germany's Axel Springer swoops for British newspaper The Telegraph
-
US sheds jobs in February in warning sign for Trump's economy

-
 Sole Iranian competitor out of Paralympics due to Middle East war
Sole Iranian competitor out of Paralympics due to Middle East war
-
Spanish PM says 'cooperation' with US should prevail over 'confrontation'

-
 Lebanese relive 'nightmare' of displacement from war
Lebanese relive 'nightmare' of displacement from war
-
US must probe Iran school strike 'very quickly', UN says

-
 AC Milan hoping to revive dimming title hopes in derby against Inter
AC Milan hoping to revive dimming title hopes in derby against Inter
-
Iceland proposes August 29 referendum on resuming EU membership talks


GEN Announces New Positive Phase 1 Trial Data of the Investigational Drug SUL-238 for Alzheimer's and Other Neurodegenerative Diseases
Phase 1 results show that SUL-238 is safe, well-tolerated, and demonstrates favorable pharmacokinetics with high CSF penetration in healthy elderly volunteers, supporting its advancement into further clinical development for Alzheimer's and other neurodegenerative diseases.
ANKARA, TR / ACCESS Newswire / December 2, 2025 / GEN Pharmaceuticals (GENIL.IS), Türkiye's leading specialty pharmaceutical company, has announced new positive results from its Phase 1 clinical trial evaluating the safety, tolerability, and pharmacokinetics (PK) of first-in-class, novel orally administered mitochondria-directed drug candidate SUL-238 in healthy elderly volunteers. The findings were presented at the 18th Clinical Trials on Alzheimer's Disease (CTAD) in San Diego, CA (United States) today.
SUL-238 was originally discovered by Sulfateq and has since been further developed through a collaborative effort of Sulfateq and GEN as a novel therapeutic in neurodegenerative diseases.
This Phase 1 randomized, double-blind, placebo-controlled study evaluated the safety, tolerability, and pharmacokinetics (PK) after multiple-ascending doses (MAD) of orally administered SUL-238 in healthy elderly men and women (aged ≥40 years). The study included two cohorts with a treatment period of 14 days and a safety follow-up through 14 days after the last dose. 15 healthy adults in each cohort were randomized in a 2:1 ratio to receive SUL-238 or placebo. Total daily dose of SUL-238 was 4000 mg (2000 mg b.i.d., first cohort) or 4500 mg (1500 mg t.i.d., second cohort). SUL-238 demonstrated an excellent safety and tolerability profile after multiple doses in both cohorts, while demonstrating a favourable PK profile and a high cerebrospinal fluid (CSF) penetration, making it a promising candidate for further clinical development in neurodegenerative diseases, including Alzheimer's and Parkinson's diseases.
Key Findings:
Safety in both cohorts:
No clinically significant changes were observed in physical and neurological exams, vital signs, ECG, and clinical laboratory parameters.
AE rates were comparable between participants receiving SUL-238 and placebo.
All AEs were of mild intensity or considered not related to SUL-238.
First cohort PK (2000 mg b.i.d.):
SUL-238 was rapidly absorbed with a mean time to maximum plasma concentration (Tmax) reached at 1.25(±0.54) and 1.50(±0.53) hours on day 1 and day 14, respectively.
Mean terminal elimination half-life (t1/2) was3.50(±1.06) hours on day 14.
Mean trough plasma concentration of SUL-238 was 39.23(±24.31) ng/mL and 41.49(±18.20) ng/mL on day 8 and day 14, respectively.
Second cohort PK (1500 mg t.i.d.):
SUL-238 was rapidly absorbed, with a mean time to maximum plasma concentration (Tmax) reached at 0.95(±0.16) and 1.00(±0.00) hours on day 1 and day 14, respectively.
Mean terminal elimination half-life (t1/2):3.74(±1.84) hours on day 14.
Mean trough plasma concentration of SUL-238 was 57.98(±31.08) and 60.63(±64.14) ng/mL on day 8 and day 14, respectively.
Abidin Gülmüş, Chairman of GEN, stated:
"We're greatly motivated by these new positive results of SUL-238 in our Phase 1 trial, which mark a key advance toward addressing Alzheimer's disease at its biological foundation."
Nadir Ulu, MD, PhD, Vice President of R&D at GEN, added:
"With its excellent safety and PK profile in this Multiple Ascending Dose Phase 1 trial, SUL-238 continues to represent a very strong drug candidate for further clinical development aimed at meeting the critical unmet needs in neurodegenerative diseases, including Alzheimer's disease.
About SUL-238
SUL-238 is a novel, first-in-class, hibernation-derived small molecule designed to target mitochondria, the 'powerhouse' of the cell. SUL-238 supports mitochondrial bioenergetics via complex I/IV activation and enhances mitochondrial function in various preclinical models for neurodegenerative, cardiovascular, and renal diseases, as well as in accelerated aging. SUL-238 exhibits the capability to cross the blood-brain barrier and has undergone extensive safety evaluation in preclinical and clinical Phase 1 studies. GEN licenses SUL-238 from Sulfateq B.V. for neurodegenerative disease applications.
About GEN:
Founded in 1998, GEN is Türkiye's leading specialty pharmaceutical company, focused on developing innovative therapies across multiple therapeutic areas. Through significant R&D investments and global collaborations, GEN is committed to advancing healthcare worldwide. The company develops and manufactures high-quality, competitive products at its GMP-certified production facility and continues its bold efforts in original drug development via two dedicated R&D centers.
About Sulfateq:
Sulfateq B.V. is an early-stage Dutch biotech company that fosters strategic collaborations with academic and industrial research centres to accelerate the development of innovative new medicines. It has developed a novel class of small molecules, the SUL-compounds, that maintain mitochondrial health.
For more information:
www.genilac.com.tr
www.sulfateqbv.com
Contact Information
Bulutay Güneş
Sr. Head of Corporate Brand
[email protected]
Fatih Gören
Investor Relations Manager
[email protected]
SOURCE: GEN İlaç ve Sağlık Ürünleri A.Ş.
View the original press release on ACCESS Newswire
T.Ziegler--VB
