-
 Volkswagen posts 1-billion-euro loss on tariffs, Porsche woes
Volkswagen posts 1-billion-euro loss on tariffs, Porsche woes
-
'Fight fire with fire': California mulls skewing electoral map

-
 Fentanyl, beans and Ukraine: Trump hails 'success' in talks with Xi
Fentanyl, beans and Ukraine: Trump hails 'success' in talks with Xi
-
'Nowhere to sleep': Melissa upends life for Jamaicans

-
 Irish octogenarian enjoys new lease on life making harps
Irish octogenarian enjoys new lease on life making harps
-
Tanzania blackout after election chaos, deaths feared

-
 G7 meets on countering China's critical mineral dominance
G7 meets on countering China's critical mineral dominance
-
Trump hails tariff, rare earth deal with Xi

-
 Court rules against K-pop group NewJeans in label dispute
Court rules against K-pop group NewJeans in label dispute
-
India's Iyer says 'getting better by the day' after lacerated spleen
-
 Yesavage fairytale carries Blue Jays to World Series brink
Yesavage fairytale carries Blue Jays to World Series brink
-
Bank of Japan keeps interest rates unchanged

-
 Impoverished Filipinos forge a life among the tombstones
Impoverished Filipinos forge a life among the tombstones
-
Jokic posts fourth straight triple-double as Nuggets rout Pelicans

-
 UN calls for end to Sudan siege after mass hospital killings
UN calls for end to Sudan siege after mass hospital killings
-
Teenage Australian cricketer dies after being hit by ball

-
 As Russia advances on Kupiansk, Ukrainians fear second occupation
As Russia advances on Kupiansk, Ukrainians fear second occupation
-
Trade truce in balance as Trump meets 'tough negotiator' Xi

-
 China to send youngest astronaut, mice on space mission this week
China to send youngest astronaut, mice on space mission this week
-
Yesavage gem carries Blue Jays to brink of World Series as Dodgers downed

-
 With inflation under control, ECB to hold rates steady again
With inflation under control, ECB to hold rates steady again
-
Asia stocks muted with all eyes on Trump-Xi meeting

-
 Personal tipping points: Four people share their climate journeys
Personal tipping points: Four people share their climate journeys
-
Moto3 rider Dettwiler 'no longer critical' after crash: family

-
 US economy in the dark as government shutdown cuts off crucial data
US economy in the dark as government shutdown cuts off crucial data
-
Trump orders nuclear testing resumption ahead of Xi talks

-
 'Utter madness': NZ farmers agree dairy sale to French group
'Utter madness': NZ farmers agree dairy sale to French group
-
Samsung posts 32% profit rise on-year in third quarter

-
 30 years after cliffhanger vote, Quebec separatists voice hope for independence
30 years after cliffhanger vote, Quebec separatists voice hope for independence
-
Taxes, labor laws, pensions: what Milei wants to do next

-
 South Sudan's blind football team dreams of Paralympic glory
South Sudan's blind football team dreams of Paralympic glory
-
US says 4 killed in new strike on alleged Pacific drug boat

-
 What we do and don't know about Rio's deadly police raid
What we do and don't know about Rio's deadly police raid
-
'They slit my son's throat' says mother of teen killed in Rio police raid

-
 Arteta hails 'special' Dowman after 15-year-old makes historic Arsenal start
Arteta hails 'special' Dowman after 15-year-old makes historic Arsenal start
-
Google parent Alphabet posts first $100 bn quarter as AI fuels growth

-
 Underwater 'human habitat' aims to allow researchers to make weeklong dives
Underwater 'human habitat' aims to allow researchers to make weeklong dives
-
Maresca slams Delap for 'stupid' red card in Chelsea win at Wolves

-
 'Non-interventionist' Trump flexes muscles in Latin America
'Non-interventionist' Trump flexes muscles in Latin America
-
Slot defends League Cup selection despite not meeting 'Liverpool standards'

-
 'Poor' PSG retain Ligue 1 lead despite stalemate and Doue injury
'Poor' PSG retain Ligue 1 lead despite stalemate and Doue injury
-
Liverpool crisis mounts after League Cup exit against Palace

-
 Kane scores twice as Bayern set European wins record
Kane scores twice as Bayern set European wins record
-
Radio Free Asia suspends operations after Trump cuts and shutdown

-
 Meta shares sink as $16 bn US tax charge tanks profit
Meta shares sink as $16 bn US tax charge tanks profit
-
Dollar rises after Fed chair says December rate cut not a given

-
 Google parent Alphabet posts first $100 bn quarter as AI drives growth
Google parent Alphabet posts first $100 bn quarter as AI drives growth
-
Rob Jetten: ex-athlete setting the pace in Dutch politics

-
 Juve bounce back after Tudor sacking as Roma keep pace with leaders Napoli
Juve bounce back after Tudor sacking as Roma keep pace with leaders Napoli
-
Favorite Sovereignty scratched from Breeders' Cup Classic after fever


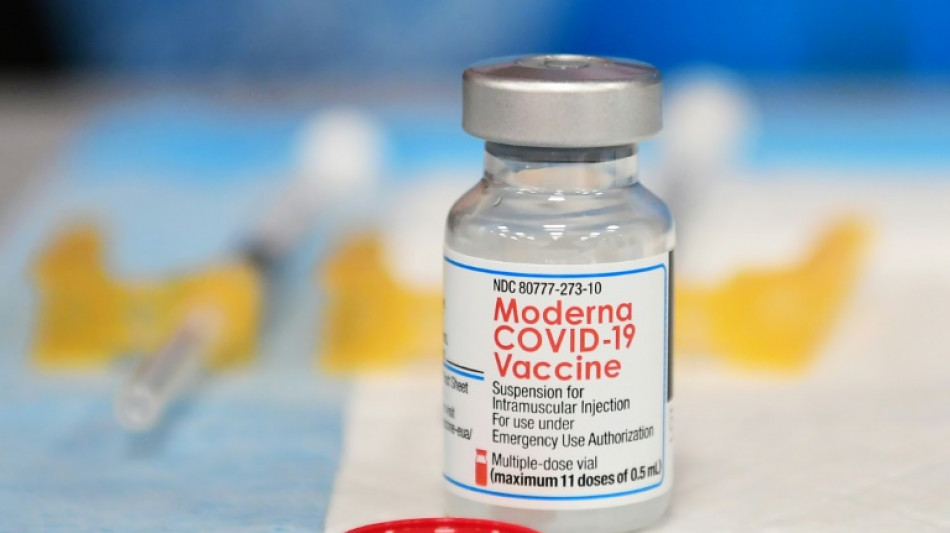
Moderna Covid vaccine gets full US approval
The US Food and Drug Administration (FDA) announced Monday that it has granted full approval to Moderna's Covid-19 "Spikevax" vaccine, which had previously received an emergency use authorization in the United States.
"The public can be assured that Spikevax meets the FDA's high standards for safety, effectiveness and manufacturing quality required of any vaccine approved for use in the United States," acting FDA commissioner Janet Woodcock said in a statement.
"The FDA's approval of Spikevax is a significant step in the fight against the Covid-19 pandemic, marking the second vaccine approved to prevent Covid-19," Woodcock said.
The full approval of the Moderna vaccine is for individuals aged 18 or older.
A Pfizer vaccine for individuals aged 16 or older received full FDA approval at the end of August.
Woodcock said she hoped FDA approval of the Moderna vaccine "may instill additional confidence in making the decision to get vaccinated."
Moderna CEO Stephane Bancel called the FDA move a "momentous milestone" for the company.
"Our Covid-19 vaccine has been administered to hundreds of millions of people around the world, protecting people from Covid-19 infection, hospitalization and death," Bancel said in a statement.
The Moderna vaccine received an emergency use authorization in December 2020.
The full approval from the FDA concerns the first two doses of the Moderna vaccine. A booster dose remains under an emergency use authorization.
The FDA said clinical trials to determine the effectiveness of Spikevax included 14,287 vaccine recipients and 14,164 placebo recipients.
The most common side effects by clinical trial participants were pain at the injection site, fatigue, headaches, muscle or joint pain, chills, nausea or vomiting and swollen lymph nodes, the FDA said.
It said there was an increased risk in men aged 18 to 24 of myocarditis -- inflammation of the heart muscle -- and pericarditis -- inflammation of tissue surrounding the heart -- following vaccination with the second dose.
But, the FDA said, it has determined that the "benefits of the vaccine outweigh the risk of myocarditis and pericarditis in individuals 18 years of age and older."
O.Krause--BTB




