-
 Spain's exiled king recounts history, scandals in wistful memoir
Spain's exiled king recounts history, scandals in wistful memoir
-
Wall Street stocks steady after positive jobs data

-
 Trump blasts Democrats as government shutdown becomes longest ever
Trump blasts Democrats as government shutdown becomes longest ever
-
Indian pilgrims find 'warm welcome' in Pakistan despite tensions

-
 Inter and AC Milan complete purchase of San Siro
Inter and AC Milan complete purchase of San Siro
-
Swedish authorities inspect worksite conditions at steel startup Stegra

-
 Keys withdraws from WTA Finals with illness
Keys withdraws from WTA Finals with illness
-
Prince Harry says proud to be British despite new life in US

-
 EU strikes last-ditch deal on climate targets as COP30 looms
EU strikes last-ditch deal on climate targets as COP30 looms
-
Stocks retreat as tech bubble fears grow
-
 Shein opens first permanent store amid heavy police presence
Shein opens first permanent store amid heavy police presence
-
West Indies edge New Zealand despite Santner brilliance

-
 French pair released by Iran await return home
French pair released by Iran await return home
-
German factory orders up but outlook still muted

-
 Death toll tops 100 as Philippines digs out after typhoon
Death toll tops 100 as Philippines digs out after typhoon
-
Attack on key city in Sudan's Kordofan region kills 40: UN

-
 'No one could stop it': Sudanese describe mass rapes while fleeing El-Fasher
'No one could stop it': Sudanese describe mass rapes while fleeing El-Fasher
-
Champagne and cheers across New York as Mamdani soars to victory

-
 Medieval tower collapse adds to Italy's workplace toll
Medieval tower collapse adds to Italy's workplace toll
-
BMW boosts profitability despite China, tariff woes

-
 South Africa's Wiese wary of 'hurt' France before re-match
South Africa's Wiese wary of 'hurt' France before re-match
-
Beyond limits: Croatian freediver's breathtaking record

-
 Tottenham supporting Udogie after alleged gun threat in London
Tottenham supporting Udogie after alleged gun threat in London
-
Thunder roll Clippers to stay unbeaten as SGA keeps streak alive

-
 In appeal, Australian mushroom murderer alleges 'miscarriage of justice'
In appeal, Australian mushroom murderer alleges 'miscarriage of justice'
-
Toyota hikes profit forecasts 'despite US tariffs'

-
 Ex-France lock Willemse challenges Meafou to become 'the bully'
Ex-France lock Willemse challenges Meafou to become 'the bully'
-
Ukrainians to honour sporting dead by building country they 'died for': minister

-
 At least 7 dead after UPS cargo plane crashes near Louisville airport
At least 7 dead after UPS cargo plane crashes near Louisville airport
-
US Supreme Court hears challenge to Trump tariff powers

-
 US government shutdown becomes longest in history
US government shutdown becomes longest in history
-
India's Modi readies bellwether poll in poorest state

-
 Green goals versus growth needs: India's climate scorecard
Green goals versus growth needs: India's climate scorecard
-
Where things stand on China-US trade after Trump and Xi talk

-
 Sri Lanka targets big fish in anti-corruption push
Sri Lanka targets big fish in anti-corruption push
-
NY elects leftist mayor on big election night for Democrats

-
 Injured Jordie Barrett to miss rest of All Blacks tour
Injured Jordie Barrett to miss rest of All Blacks tour
-
Asian markets tumble as tech bubble fears grow
-
 Pay to protect: Brazil pitches new forest fund at COP30
Pay to protect: Brazil pitches new forest fund at COP30
-
Iraq's social media mercenaries dying for Russia
-
 Young leftist Trump foe elected New York mayor
Young leftist Trump foe elected New York mayor
-
Concerns at ILO over expected appointment of close Trump advisor

-
 Venus Williams to return to Auckland Classic at the age of 45
Venus Williams to return to Auckland Classic at the age of 45
-
No deal yet on EU climate targets as COP30 looms

-
 Typhoon death toll climbs to 66 in the Philippines
Typhoon death toll climbs to 66 in the Philippines
-
NATO tests war preparedness on eastern flank facing Russia

-
 Uncapped opener Weatherald in Australia squad for first Ashes Test
Uncapped opener Weatherald in Australia squad for first Ashes Test
-
Liverpool down Real Madrid in Champions League, Bayern edge PSG

-
 Van Dijk tells Liverpool to keep calm and follow Arsenal's lead
Van Dijk tells Liverpool to keep calm and follow Arsenal's lead
-
PSG left to sweat on injuries to Dembele and Hakimi


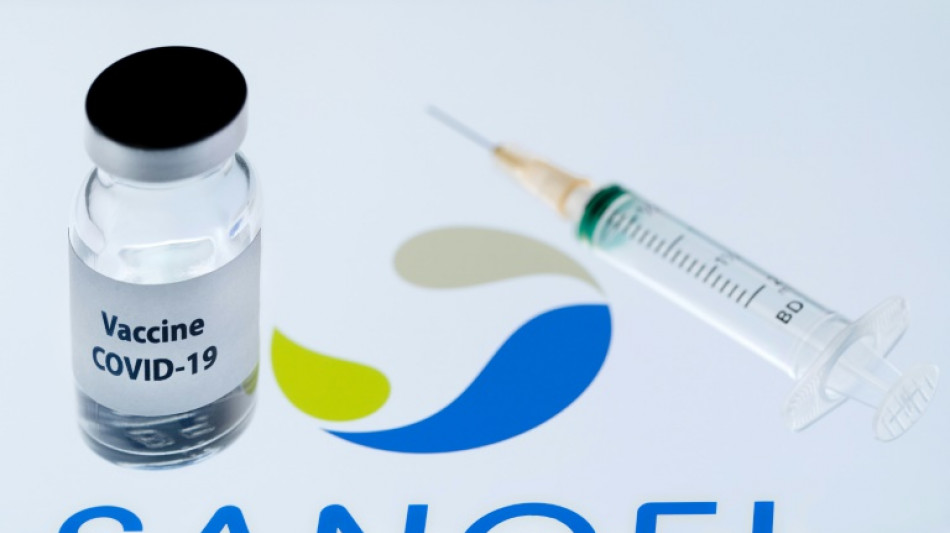
EU watchdog backs Sanofi Covid booster jab
The EU on Thursday approved a Covid booster vaccine by French drug maker Sanofi and Britain's GSK after it gave positive results against the Omicron variant in trials.
The approval of the "next generation" jab by the European Medicines Agency (EMA) is a shot in the arm for Sanofi and GSK, which have lagged behind rivals in offering a vaccine.
The VidPrevtyn Beta jab could be used as a booster in adults previously given mRNA jabs like those from Pfizer/BioNTech and Moderna, or viral vector vaccines made by AstraZeneca and Johnson & Johnson, the EMA said.
"A booster dose of VidPrevtyn Beta is expected to be at least as effective as Comirnaty (Pfizer's vaccine) at restoring protection against Covid-19," the Amsterdam-based EMA said.
A trial of 162 adults given the Sanofi-GSK booster showed that it triggers a higher production of antibodies against the Omicron BA.1 subvariant than Pfizer's jab, the regulator said.
A second study restored immunity in 627 adults who received other vaccines for their first course of jabs.
Sanofi said it was ready to start its first shipments of the booster in Britain and the EU, in line with advance contracts for 70 million doses.
"Today's approval validates our research in developing a novel solution for the Covid-19 pandemic," Thomas Triomphe, Sanofi executive vice president for vaccines, said.
The vaccine combines a Sanofi-developed antigen based on the Beta variant, which stimulates the production of germ-killing antibodies, with GSK's adjuvant technology, a substance that bolsters the immune response triggered by a vaccine.
- End of a long road -
Sanofi and GSK developed the jab at the same time that they are waiting for regulatory approval for their first-generation vaccine.
The approval marks the end of a long journey for Sanofi to bring a Covid vaccine to market. The French pharma giant, considered to be a world leader on vaccines, has come under huge scrutiny at home for failing to roll out a Covid jab earlier.
The firm vowed to produce a billion vaccine doses in 2021, only for a dosage problem during clinical trials to send its researchers back to the drawing board. It also tried to develop a vaccine based on mRNA technology, only to abandon that plan as well.
While it struggled, Pfizer/BioNTech and Moderna brought their vaccines to market at a pace never before seen in history. Both vaccines were approved nearly two full years before Sanofi's breakthrough on Thursday.
"It is, it must be recognised, a failure... compared to the speed that was needed," Sanofi chairman Serge Weinberg told a shareholders' meeting in May.
While Sanofi has finally managed to get a Covid vaccine approved, the question remains about how much demand remains in an already crowded market.
Last week frontrunner Pfizer raised the annual sales forecast for its vaccine to $36 billion on the back of new deals for boosters.
Moderna meanwhile slashed the sales forecast for its own vaccine by $2-$3 billion dollars due to shipment delays.
On Thursday, French-Austrian biotech Valneva announced it will cut up to a quarter of its workforce.
Valneva became the first French firm to get a Covid vaccine approved by the EMA in June. It suspended production a month later, however, after the EU slashed its initial order of 60 million doses to just 1.25 million.
K.Thomson--BTB




