
-
 BMW reports rising profitability, shares jump
BMW reports rising profitability, shares jump
-
Bolivia Supreme Court orders release of jailed ex-president Jeanine Anez

-
 Wall Street stocks rise after positive jobs data
Wall Street stocks rise after positive jobs data
-
'Hostage diplomacy': longstanding Iran tactic presenting dilemma for West

-
 Rybakina stays perfect at WTA Finals with win over alternate Alexandrova
Rybakina stays perfect at WTA Finals with win over alternate Alexandrova
-
Le Garrec welcomes Dupont help in training for Springboks showdown

-
 Brussels wants high-speed rail linking EU capitals by 2040
Brussels wants high-speed rail linking EU capitals by 2040
-
Swiss business chiefs met Trump on tariffs: Bern

-
 Spain's exiled king recounts history, scandals in wistful memoir
Spain's exiled king recounts history, scandals in wistful memoir
-
Wall Street stocks steady after positive jobs data

-
 Trump blasts Democrats as government shutdown becomes longest ever
Trump blasts Democrats as government shutdown becomes longest ever
-
Indian pilgrims find 'warm welcome' in Pakistan despite tensions

-
 Inter and AC Milan complete purchase of San Siro
Inter and AC Milan complete purchase of San Siro
-
Swedish authorities inspect worksite conditions at steel startup Stegra

-
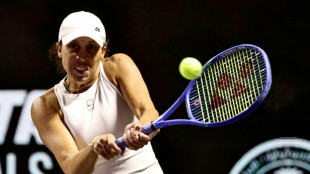 Keys withdraws from WTA Finals with illness
Keys withdraws from WTA Finals with illness
-
Prince Harry says proud to be British despite new life in US

-
 EU strikes last-ditch deal on climate targets as COP30 looms
EU strikes last-ditch deal on climate targets as COP30 looms
-
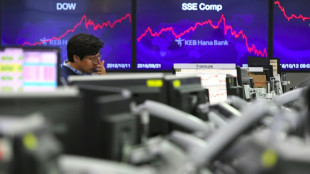
Stocks retreat as tech bubble fears grow
-
 Shein opens first permanent store amid heavy police presence
Shein opens first permanent store amid heavy police presence
-
West Indies edge New Zealand despite Santner brilliance

-
 French pair released by Iran await return home
French pair released by Iran await return home
-
German factory orders up but outlook still muted

-
 Death toll tops 100 as Philippines digs out after typhoon
Death toll tops 100 as Philippines digs out after typhoon
-
Attack on key city in Sudan's Kordofan region kills 40: UN

-
 'No one could stop it': Sudanese describe mass rapes while fleeing El-Fasher
'No one could stop it': Sudanese describe mass rapes while fleeing El-Fasher
-
Champagne and cheers across New York as Mamdani soars to victory

-
 Medieval tower collapse adds to Italy's workplace toll
Medieval tower collapse adds to Italy's workplace toll
-
BMW boosts profitability despite China, tariff woes

-
 South Africa's Wiese wary of 'hurt' France before re-match
South Africa's Wiese wary of 'hurt' France before re-match
-
Beyond limits: Croatian freediver's breathtaking record

-
 Tottenham supporting Udogie after alleged gun threat in London
Tottenham supporting Udogie after alleged gun threat in London
-
Thunder roll Clippers to stay unbeaten as SGA keeps streak alive

-
 In appeal, Australian mushroom murderer alleges 'miscarriage of justice'
In appeal, Australian mushroom murderer alleges 'miscarriage of justice'
-
Toyota hikes profit forecasts 'despite US tariffs'

-
 Ex-France lock Willemse challenges Meafou to become 'the bully'
Ex-France lock Willemse challenges Meafou to become 'the bully'
-
Ukrainians to honour sporting dead by building country they 'died for': minister

-
 At least 7 dead after UPS cargo plane crashes near Louisville airport
At least 7 dead after UPS cargo plane crashes near Louisville airport
-
US Supreme Court hears challenge to Trump tariff powers

-
 US government shutdown becomes longest in history
US government shutdown becomes longest in history
-
India's Modi readies bellwether poll in poorest state

-
 Green goals versus growth needs: India's climate scorecard
Green goals versus growth needs: India's climate scorecard
-
Where things stand on China-US trade after Trump and Xi talk

-
 Sri Lanka targets big fish in anti-corruption push
Sri Lanka targets big fish in anti-corruption push
-
NY elects leftist mayor on big election night for Democrats

-
 Injured Jordie Barrett to miss rest of All Blacks tour
Injured Jordie Barrett to miss rest of All Blacks tour
-
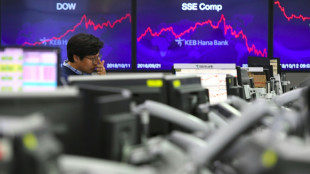
Asian markets tumble as tech bubble fears grow
-
 Pay to protect: Brazil pitches new forest fund at COP30
Pay to protect: Brazil pitches new forest fund at COP30
-
Iraq's social media mercenaries dying for Russia
-
 Young leftist Trump foe elected New York mayor
Young leftist Trump foe elected New York mayor
-
Concerns at ILO over expected appointment of close Trump advisor


US approves first vaccine against chikungunya virus
US health authorities on Thursday approved the world's first vaccine for chikungunya, a virus spread by infected mosquitoes that the Food and Drug Administration called "an emerging global health threat."
The vaccine, developed by Europe's Valneva which will be marketed under the name Ixchiq, was approved for people 18 and over who are at increased risk of exposure, the FDA said.
Ixchiq's green-light by the US drug regulator is expected to speed the vaccine's rollout in countries where the virus is most prevalent.
Chikungunya, which causes fever and severe joint pain, is generally seen in tropical and subtropical regions of Africa, southeast Asia and parts of the Americas.
"However, chikungunya virus has spread to new geographical areas causing a rise in global prevalence of the disease," the FDA said, reporting more than five million cases in the past 15 years.
"Infection with chikungunya virus can lead to severe disease and prolonged health problems, particularly for older adults and individuals with underlying medical conditions," senior FDA official Peter Marks said in a statement.
"Today's approval addresses an unmet medical need and is an important advancement in the prevention of a potentially debilitating disease with limited treatment options."
Symptoms can sometimes last for months or even years, but the virus is rarely fatal. There is currently no specific drug to treat chikungunya, aside from common medications for pain and fever relief.
In the absence of preventative treatment, until now the only way to protect against infection was to avoid getting bitten.
The vaccine is injected in one dose and contains a live, weakened version of the chikungunya virus, as is standard with other vaccines.
Two clinical trials were carried out in North America on 3,500 people. Headache, fatigue, muscle and joint pain, fever and nausea were commonly reported side effects.
Serious reactions were reported in 1.6 percent of Ixchiq recipients in the trials, with two requiring hospitalization.
Some vaccine recipients had chikungunya-like adverse reactions that lasted for 30 days or more.
Chikungunya can be passed from a pregnant person to their unborn child, and the virus can be fatal to newborns.
The FDA in its statement noted it was not known whether the vaccine virus can be transmitted from mother to a baby in utero, nor if the vaccine can cause adverse effects in newborns.
Since chikungunya was first identified in Tanzania in 1952, it has been recorded in more than 110 countries, according to the World Health Organization.
Public health experts have expressed concerns that chikungunya could be a potential future pandemic threat as climate change pushes the mosquitoes that spread it into new regions.
An application for authorization has also been filed by Valneva with the European Medicines Agency (EMA).
P.Vogel--VB




