-
 Scandic Trust Group strengthens sales network with First Idea Consultant
Scandic Trust Group strengthens sales network with First Idea Consultant
-
MotoGP legend Agostini admires Marc Marquez's 'desire to win'

-
 Nepal searches for avalanche victims
Nepal searches for avalanche victims
-
Hezbollah rejects any negotiations between Lebanon and Israel

-
 Chapman blitz leads Black Caps to tight T20 victory over West Indies
Chapman blitz leads Black Caps to tight T20 victory over West Indies
-
France urges EU to sanction Shein platform

-
 France opt for Le Garrec as Dupont replacement for South Africa Test
France opt for Le Garrec as Dupont replacement for South Africa Test
-
Turmoil in tiaras at Miss Universe pageant in Thailand

-
 Probe into Thales defence group looking at Indonesian contract
Probe into Thales defence group looking at Indonesian contract
-
US to cancel flights as longest govt shutdown drags on

-
 Home in Nigeria, ex-refugees find themselves in a war zone
Home in Nigeria, ex-refugees find themselves in a war zone
-
Doncic's Lakers hold off Wembanyama's Spurs, Blazers silence Thunder

-
 For Turkey's LGBTQ community, draft law sparks existential alarm
For Turkey's LGBTQ community, draft law sparks existential alarm
-
Musk's $1 trillion pay package to face Tesla shareholder vote

-
 Tonga rugby league star out of intensive care after seizure
Tonga rugby league star out of intensive care after seizure
-
Argentine ex-president Kirchner goes on trial in new corruption case

-
 Dams, housing, pensions: Franco disinformation flourishes online
Dams, housing, pensions: Franco disinformation flourishes online
-
Endo returns as Japan look to build on Brazil win

-
 Franco captivates young Spaniards 50 years after death
Franco captivates young Spaniards 50 years after death
-
German steel industry girds for uncertain future

-
 IPL champions Bengaluru could be sold for 'as much as $2 billion'
IPL champions Bengaluru could be sold for 'as much as $2 billion'
-
Budget impasse threatens Belgium's ruling coalition

-
 New Zealand ex-top cop admits to having material showing child abuse, bestiality
New Zealand ex-top cop admits to having material showing child abuse, bestiality
-
BoE set for finely balanced pre-budget rate call

-
 Australian kingpin obtains shorter sentence over drug charge
Australian kingpin obtains shorter sentence over drug charge
-
Weatherald's unenviable Ashes task: fill giant hole at top left by Warner

-
 Ovechkin first to score 900 NHL goals as Capitals beat Blues
Ovechkin first to score 900 NHL goals as Capitals beat Blues
-
On Mexico City's streets, vendors fight to make it to World Cup

-
 Asian markets bounce from selloff as US jobs beat forecasts
Asian markets bounce from selloff as US jobs beat forecasts
-
Philippine death toll tops 140 as typhoon heads towards Vietnam

-
 Kyrgios targets 'miracle' Australian Open return after knee improves
Kyrgios targets 'miracle' Australian Open return after knee improves
-
'AI president': Trump deepfakes glorify himself, trash rivals

-
 Belgium probes drone sightings after flights halted overnight
Belgium probes drone sightings after flights halted overnight
-
Five things to know about 'forest COP' host city Belem

-
 World leaders to rally climate fight ahead of Amazon summit
World leaders to rally climate fight ahead of Amazon summit
-
Engine fell off US cargo plane before deadly crash: officials

-
 Mexican leader calls for tougher sexual harassment laws after attack
Mexican leader calls for tougher sexual harassment laws after attack
-
Meghan Markle set for big screen return: reports

-
 Japan deploys troops after wave of deadly bear attacks
Japan deploys troops after wave of deadly bear attacks
-
FIFA announce new peace prize to be awarded at World Cup draw in Washington

-
 Australia's Cummins hints at return for second Ashes Test
Australia's Cummins hints at return for second Ashes Test
-
Boeing settles with one plaintiff in 737 MAX crash trial

-
 Man City win as Inter stay perfect, Barca held in Champions League
Man City win as Inter stay perfect, Barca held in Champions League
-
French superstar DJ Snake wants new album to 'build bridges'

-
 Barca rescue draw at Club Brugge in six-goal thriller
Barca rescue draw at Club Brugge in six-goal thriller
-
Foden hits top form as Man City thrash Dortmund

-
 NBA officials brief Congress committee over gambling probe
NBA officials brief Congress committee over gambling probe
-
Inter beat Kairat Almaty to maintain Champions League perfection

-
 Newcastle sink Bilbao to extend Champions League winning run
Newcastle sink Bilbao to extend Champions League winning run
-
Wall Street stocks rebound after positive jobs data


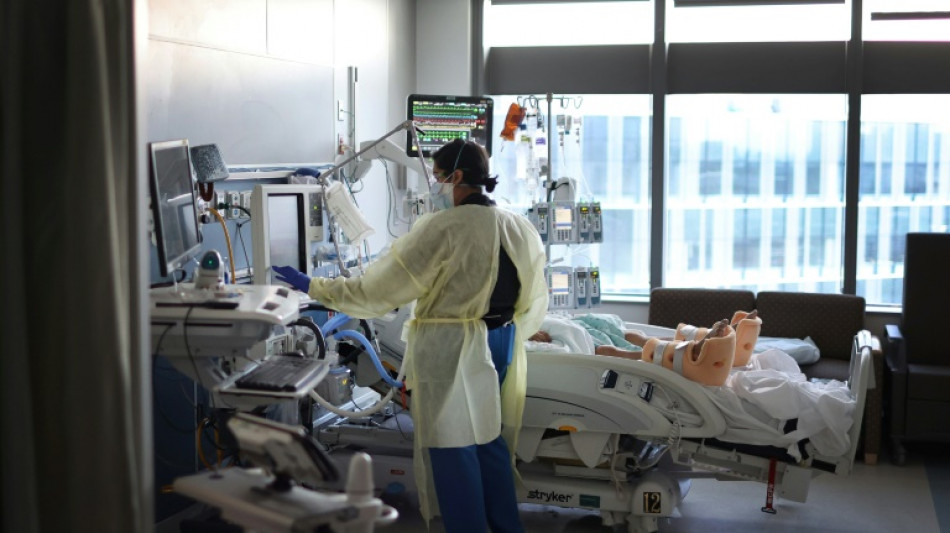
Trial of new Covid treatment yields encouraging results: study
A single-injection antiviral treatment for newly-infected Covid-19 patients reduced the risk of hospitalization by half in a large-scale clinical trial, according to a study published on Wednesday.
Stanford University professor Jeffrey Glenn, coauthor of the study published in the New England Journal of Medicine (NEJM), said the new drug "showed profound benefits for vaccinated and unvaccinated people alike."
While the number of Americans dying daily of coronavirus has fallen to around 500, treatments for the disease remain limited. One of the most common, Paxlovid made by Pfizer, involves taking 30 pills over five days.
The new treatment involves a single dose of pegylated lambda-interferon, a synthetic version of a naturally occurring protein that infected cells secrete to defend against viral infection.
"What it does is it binds receptors on the surfaces of cells that activate our own antiviral defense mechanisms," said Glenn, a professor of medicine, microbiology and immunology who heads the Stanford Biosecurity and Pandemic Preparedness Initiative.
"So if a virus has infected the cell it will turn on processes that aim to destroy the virus's replication," he said. "It will also send signals to neighboring cells to warn them viruses are on their way and get ready to defend yourself."
Receptors for interferon lambda are primarily in the linings of the lungs, airways and intestine -- the main places Covid strikes.
"We're turning on these antiviral mechanisms in the cells, the lung, where the infection is happening," Glenn said.
The Phase 3 trial of the drug, conducted between June 2021 and February 2022, involved nearly 2,000 patients with Covid symptoms in Brazil and Canada, about 85 percent of whom had been vaccinated.
A total of 931 newly-infected Covid patients were given a single injection of interferon lambda while 1,018 participants were given a placebo.
The risk of Covid-19–related hospitalization or death from any cause was 47 percent lower in the interferon group than in the placebo group, according to the researchers.
Twenty-five of the 931 people who received the injection within seven days of exhibiting Covid symptoms were hospitalized compared with 57 of the 1,018 who received the placebo.
Vaccinated patients treated with interferon lambda experienced a 51 percent reduction in hospitalization relative to the placebo group.
There was an 89 percent reduction in hospitalization among unvaccinated patients treated within the first three days of the onset of Covid symptoms compared with the placebo group.
- Developed for hepatitis D -
Glenn said interferon lambda proved effective against all Covid variants tested, including Omicron, and side effects in the group receiving the injections were no greater than among the placebo recipients.
Glenn is the founder of a small biotechnology company called Eiger Biopharmaceuticals that acquired interferon lambda to develop drugs for hepatitis delta virus.
"When Covid came, I said this would be the perfect drug for Covid," said Glenn, who left the Palo Alto-based company but remains on the board of directors and is an equity holder.
Eiger sought an emergency use authorization for interferon lambda from the US Federal Drug Administration (FDA) for Covid treatment last year but it was not granted.
That was "very frustrating," Glenn said, but he was hopeful publication of the study in the NEJM "will help encourage regulators here and around the world to find a way to get lambda into patients as soon as possible."
C.Meier--BTB




