-
 McLaughlin-Levrone, Russell book World Championship berths at US trials
McLaughlin-Levrone, Russell book World Championship berths at US trials
-
Rybakina outlasts Yastremska to reach WTA Montreal quarter-finals

-
 Young seizes five-stroke lead at PGA Wyndham Championship
Young seizes five-stroke lead at PGA Wyndham Championship
-
Rescuers recover body of trapped worker at Chile copper mine

-
 Patrick Star and 'Drag Queen' crab: underwater robot live stream captivates Argentines
Patrick Star and 'Drag Queen' crab: underwater robot live stream captivates Argentines
-
McLaughlin-Levrone wins 400m to seal World Championship berth

-
 Khachanov downs Ruud to book ATP Toronto clash with Michelsen
Khachanov downs Ruud to book ATP Toronto clash with Michelsen
-
Young Catholics give rock star welcome to Pope Leo at vigil

-
 Yamashita's lead in Women's British Open cut to one shot
Yamashita's lead in Women's British Open cut to one shot
-
Rovanpera survives puncture to close in on home win in Finland Rally

-
 Siraj strikes after Jaiswal helps India set England daunting target
Siraj strikes after Jaiswal helps India set England daunting target
-
Doncic inks three-year $165 mln Lakers extension

-
 Hamilton feeling 'useless' after Hungarian GP qualifying flop
Hamilton feeling 'useless' after Hungarian GP qualifying flop
-
Elation as pope arrives by helicopter to open-air youth vigil in Rome

-
 McLaren blown away by changing wind as Leclerc lands pole for Ferrari
McLaren blown away by changing wind as Leclerc lands pole for Ferrari
-
Home hero Ferrand-Prevot in epic climb to Tour de France lead

-
 Leclerc ends Ferrari barren run with stunning pole ahead of McLarens
Leclerc ends Ferrari barren run with stunning pole ahead of McLarens
-
Ferrari's Leclerc on pole for Hungarian GP

-
 Jaiswal's hundred leaves England needing Oval-record chase to beat India
Jaiswal's hundred leaves England needing Oval-record chase to beat India
-
At open-air Church party, many thousands of young Catholics eagerly await pope

-
 Schmidt hails 'grit and resilience' as his Wallabies upset Lions
Schmidt hails 'grit and resilience' as his Wallabies upset Lions
-
Dmitry Medvedev: Russia's hawkish ex-president

-
 Imperious Ledecky beats McIntosh to win 800m free thriller
Imperious Ledecky beats McIntosh to win 800m free thriller
-
Ledecky reigns over McIntosh as record-breaking US hit back at critics

-
 Farrell says 'dream' Lions should be proud despite bitter defeat
Farrell says 'dream' Lions should be proud despite bitter defeat
-
Ledecky beats McIntosh to win 800m freestyle thriller

-
 Fearless Wallabies stun weary Lions to win third Test 22-12
Fearless Wallabies stun weary Lions to win third Test 22-12
-
Jaiswal and Deep keep India in the hunt against England

-
 Piastri edges Norris as McLaren dominate Hungarian GP final practice
Piastri edges Norris as McLaren dominate Hungarian GP final practice
-
US envoy meets Israeli hostage families in Tel Aviv

-
 McKeown beats Smith again for world backstroke double
McKeown beats Smith again for world backstroke double
-
New dad McEvoy adds 'unreal' world swimming gold to Olympic title

-
 Walsh completes world butterfly double in riposte to Phelps
Walsh completes world butterfly double in riposte to Phelps
-
Turkey starts supplying Azerbaijani gas to boost Syria's power output

-
 Thousands of young Catholics converge for grand Pope Leo vigil
Thousands of young Catholics converge for grand Pope Leo vigil
-
SpaceX Crew Dragon docks with International Space Station

-
 US do talking in pool after Phelps, Lochte slam worlds performance
US do talking in pool after Phelps, Lochte slam worlds performance
-
Up to a million young Catholics expected for grand Pope Leo vigil

-
 New push to reach plastic polution pact
New push to reach plastic polution pact
-
Second seed Fritz ends Canadian hopes at ATP Toronto Masters

-
 Japan sweats through hottest July on record
Japan sweats through hottest July on record
-
Jefferson-Wooden, Bednarek blaze to 100m titles at US trials

-
 Son Heung-min to leave Tottenham this summer after decade
Son Heung-min to leave Tottenham this summer after decade
-
Bid to relocate US Space Shuttle Discovery faces museum pushback

-
 Academics warn Columbia University deal sets dangerous precedent
Academics warn Columbia University deal sets dangerous precedent
-
Sevastova topples Pegula to book date with Osaka, Swiatek advances in Montreal

-
 Former Olympic champion Mu-Nikolayev fails in worlds bid
Former Olympic champion Mu-Nikolayev fails in worlds bid
-
Sensible and steely: how Mexico's Sheinbaum has dealt with Trump

-
 Young leads at weather-hit PGA Wyndham Championship
Young leads at weather-hit PGA Wyndham Championship
-
US sprint star Richardson out of trials following arrest

| RBGPF | 0% | 74.94 | $ | |
| SCU | 0% | 12.72 | $ | |
| CMSD | 0.34% | 23.35 | $ | |
| CMSC | 0.09% | 22.87 | $ | |
| GSK | 1.09% | 37.56 | $ | |
| SCS | -1.47% | 10.18 | $ | |
| NGG | 1.99% | 71.82 | $ | |
| RELX | -0.58% | 51.59 | $ | |
| BTI | 1.23% | 54.35 | $ | |
| RIO | -0.2% | 59.65 | $ | |
| AZN | 1.16% | 73.95 | $ | |
| BCC | -0.55% | 83.35 | $ | |
| JRI | -0.23% | 13.1 | $ | |
| VOD | 1.37% | 10.96 | $ | |
| BP | -1.26% | 31.75 | $ | |
| RYCEF | 0.07% | 14.19 | $ | |
| BCE | 1.02% | 23.57 | $ |

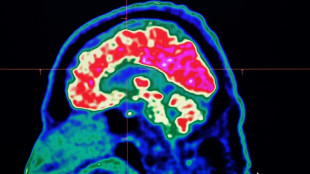
Revolution or mirage? Controversy surrounds new Alzheimer's drugs
Two new drugs, the first capable of slowing down the debilitating progression of Alzheimer's disease, have become embroiled in one of the biggest medical controversies in recent years.
For their defenders, the drugs lecanemab and donanemab represent the first real chance to fight the disease after decades of research -- for detractors, they are another disappointment after a long line of costly failures.
"We have turned a corner" thanks to these treatments, British biologist John Hardy, who has been studying Alzheimer's since the 1990s, told AFP.
Rob Howard, a professor of old age psychiatry at University College London, was on the other side.
"I think that the drugs have been used to raise false and unrealistic hopes in people with Alzheimer's disease and their families," he said.
These opposing statements sum up the entrenched positions on the recently introduced drugs for Alzheimer's, the most common form of dementia which millions of people across the world suffer from.
Lecanemab, sold under the name Leqembi, was developed by US pharma firms Biogen and Eisai. Donanemab, developed by pharma giant Eli Lilly, is sold as Kisunla.
The controversy has seen countries take different stances on whether to approve the drugs or not.
The United States gave the green light to lecanemab in 2023, then donanemab earlier this year.
However the European Union rejected lecanemab in July, a bad omen for donanemab's chance of approval.
Last month, the UK steered a middle course, approving the use of lecanemab but not making it available on the state National Health Service.
What no one denies is that the two drugs are the most effective Alzheimer's treatments ever -- but their effectiveness is limited.
Both appear to reduce cognitive decline in patients at the onset of their disease by around 30 percent.
While that may seem high, it represents a relatively small difference over the year-and-a-half period when the studies were carried out.
"The benefits are so tiny as to be practically invisible in an individual patient," Howard said.
- Exorbitant cost -
For critics, there are not enough benefits to outweigh the risks of the drugs, which can sometimes cause brain swelling or bleeding that in rare cases has proved fatal.
And they are very expensive. At the prices being charged by Biogen and Eisai in the United States, lecanemab would cost 133 billion euros ($148 billion) if given to all eligible patients in the EU, according to a 2023 study.
Advocates of the drugs, including many neurologists, believe they can offer patients a few more precious months of autonomy.
They also believe that the effectiveness of the drugs could be multiplied if patients started taking them earlier in the disease's progression. This could soon be more practicable as research on diagnosing Alzheimer's more quickly has recently been making significant strides.
The differing national policies could also mean that poorer patients are left behind.
"We will see rich people going to the US" for the drugs, Hardy said.
The debate can be traced back in part to a seminal 1992 article by Hardy about how the disease actually works.
The article argues that clumps of protein called amyloid plaques -- a constant in the brains of Alzheimer's patients -- are not just one element among others, but the main factor triggering the disease.
Over the decades, many drugs targeting these amyloid plaques were developed, all of which failed -- until lecanemab and donanemab.
- Pressure from families -
The scepticism from some quarters about the new drugs could be because the previous ones were defended and even lauded by some, despite their ineffectiveness.
Christian Guy-Coichard, the head of French organisation Formindep which monitors medical conflicts of interest, accused Alzheimer's groups, researchers and pharmaceutical firms of being too close.
But France Alzheimer deputy director Benoit Durand said that very little of its funding came from Biogen/Eisai or Eli Lilly, instead pointing towards pressure for new treatments from patients' families.
"They don't understand" the EU's decision to turn down a breakthrough new drug, Durand told AFP. He also feared that laboratories could lose interest in Alzheimer's disease due to the setbacks.
Even within the pharmaceutical industry, some admit that past failures have not necessarily helped build trust.
A doctor working for Eli Lilly, who spoke on condition of anonymity, blamed its rival Biogen for overstating the benefits of previous treatment Aduhelm. The drug was controversially approved in the US in 2021 before being withdrawn.
"The Aduhelm studies were a mess," the doctor said.
The aftermath "did a lot of harm and sowed chaos in the discipline", the doctor added, pointing the finger at Biogen.
In response, Biogen told AFP that it was complying with "the principles of scientific research as well as legal and regulatory requirements".
But the Eli Lilly doctor defended the new treatments all the same, urging people to look to the future, not the past.
Like other specialists, he also acknowledged that other mechanisms besides amyloids that could be behind Alzheimer's need to be explored.
Given the disease's complexity, it is unlikely that "single-target treatments will achieve substantially larger effects" than lecanemab and donanemab, a group of experts wrote in the Journal of Prevention of Alzheimer's Disease last month.
But the new drugs are a "critical step" in Alzheimer's treatment, they added.
F.Fehr--VB