
-
 India's Modi readies bellwether poll in poorest state
India's Modi readies bellwether poll in poorest state
-
Green goals versus growth needs: India's climate scorecard

-
 Where things stand on China-US trade after Trump and Xi talk
Where things stand on China-US trade after Trump and Xi talk
-
Sri Lanka targets big fish in anti-corruption push

-
 NY elects leftist mayor on big election night for Democrats
NY elects leftist mayor on big election night for Democrats
-
Injured Jordie Barrett to miss rest of All Blacks tour

-
 Asian markets tumble as tech bubble fears grow
Asian markets tumble as tech bubble fears grow
-
Pay to protect: Brazil pitches new forest fund at COP30

-
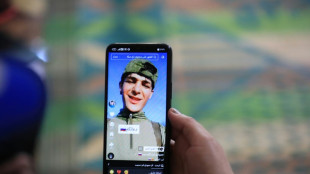 Iraq's social media mercenaries dying for Russia
Iraq's social media mercenaries dying for Russia
-
Young leftist Trump foe elected New York mayor

-
 Concerns at ILO over expected appointment of close Trump advisor
Concerns at ILO over expected appointment of close Trump advisor
-
Venus Williams to return to Auckland Classic at the age of 45

-
 No deal yet on EU climate targets as COP30 looms
No deal yet on EU climate targets as COP30 looms
-
Typhoon death toll climbs to 66 in the Philippines

-
 NATO tests war preparedness on eastern flank facing Russia
NATO tests war preparedness on eastern flank facing Russia
-
Uncapped opener Weatherald in Australia squad for first Ashes Test

-
 Liverpool down Real Madrid in Champions League, Bayern edge PSG
Liverpool down Real Madrid in Champions League, Bayern edge PSG
-
Van Dijk tells Liverpool to keep calm and follow Arsenal's lead

-
 PSG left to sweat on injuries to Dembele and Hakimi
PSG left to sweat on injuries to Dembele and Hakimi
-
Reddit, Kick to be included in Australia's social media ban

-
 Ex-Zimbabwe cricket captain Williams treated for 'drug addiction'
Ex-Zimbabwe cricket captain Williams treated for 'drug addiction'
-
Padres ace Darvish to miss 2026 MLB season after surgery

-
 Diaz hero and villain as Bayern beat PSG in Champions League showdown
Diaz hero and villain as Bayern beat PSG in Champions League showdown
-
Liverpool master Real Madrid on Alexander-Arnold's return

-
 Van de Ven back in favour as stunning strike fuels Spurs rout
Van de Ven back in favour as stunning strike fuels Spurs rout
-
Juve held by Sporting Lisbon in stalling Champions League campaign

-
 New lawsuit alleges Spotify allows streaming fraud
New lawsuit alleges Spotify allows streaming fraud
-
Stocks mostly drop as tech rally fades

-
 LIV Golf switching to 72-hole format in 2026: official
LIV Golf switching to 72-hole format in 2026: official
-
Manchester City have become 'more beatable', says Dortmund's Gross

-
 Merino brace sends Arsenal past Slavia in Champions League
Merino brace sends Arsenal past Slavia in Champions League
-
Djokovic makes winning return in Athens

-
 Napoli and Eintracht Frankfurt in Champions League stalemate
Napoli and Eintracht Frankfurt in Champions League stalemate
-
Arsenal's Dowman becomes youngest-ever Champions League player

-
 Cheney shaped US like no other VP. Until he didn't.
Cheney shaped US like no other VP. Until he didn't.
-
Pakistan edge South Africa in tense ODI finish in Faisalabad

-
 Brazil's Lula urges less talk, more action at COP30 climate meet
Brazil's Lula urges less talk, more action at COP30 climate meet
-
Barca's Lewandowski says his season starting now after injury struggles

-
 Burn urges Newcastle to show their ugly side in Bilbao clash
Burn urges Newcastle to show their ugly side in Bilbao clash
-
French pair released after 3-year Iran jail ordeal

-
 Getty Images largely loses lawsuit against UK AI firm
Getty Images largely loses lawsuit against UK AI firm
-
Cement maker Lafarge on trial in France over jihadist funding

-
 Sculpture of Trump strapped to a cross displayed in Switzerland
Sculpture of Trump strapped to a cross displayed in Switzerland
-
Pakistan's Rauf and Indian skipper Yadav punished over Asia Cup behaviour

-
 Libbok welcomes 'healthy' Springboks fly-half competition
Libbok welcomes 'healthy' Springboks fly-half competition
-
Reeling from earthquakes, Afghans fear coming winter

-
 Ronaldo reveals emotional retirement will come 'soon'
Ronaldo reveals emotional retirement will come 'soon'
-
Munich's surfers stunned after famed river wave vanishes

-
 Iran commemorates storming of US embassy with missile replicas, fake coffins
Iran commemorates storming of US embassy with missile replicas, fake coffins
-
Gauff sweeps Paolini aside to revitalise WTA Finals defence


Highly awaited Alzheimer's drug hit by delays
Eli Lilly's highly anticipated Alzheimer's drug has been held back for further review by regulators, the US pharmaceutical giant said Friday, in a blow for patients with the devastating brain disorder.
Donanemab has been found to slow cognitive decline in the early stages of the disease during a clinical trial -- but there was also a high rate of side effects, including deaths.
The Food and Drug Administration (FDA) "has informed Lilly it wants to further understand topics related to evaluating the safety and efficacy of donanemab," the company said in a statement Friday.
The regulator told the Indiana-based company it would convene a new meeting of experts, but hadn't provided a firm date. "As a result, the timing of expected FDA action on donanemab will be delayed beyond the first quarter of 2024."
"We are confident in donanemab's potential to offer very meaningful benefits to people with early symptomatic Alzheimer's disease," said Anne White, the company's executive vice president.
She added the FDA's decision to have a new meeting was "unexpected," but "We will work with the FDA and the stakeholders in the community to make that presentation and answer all questions."
Donanemab is an intravenously injected antibody that targets the build up beta-amyloid, a protein found in the brains of many patients with Alzheimer's.
Another anti-amyloid therapy called Leqembi, which was developed by Eisai of Japan and Biogen of Massachusetts, was granted full approval by the FDA last July and is now accessible through government-run health insurance for the elderly called Medicare.
- Slows decline, but risky -
In a paper published in the Journal of the American Medical Association last year, researchers found donanemab slowed cognitive and functional decline in patients who have early symptoms of the disease.
Forty-seven percent of those who received the drug showed no signs of cognitive decline after one year of treatment, compared to 29 percent who received a placebo.
Serious adverse events, including brain bleeds, occurred in 17.4 percent of those who received donanemab and 15.8 percent of those who received a placebo.
There were also four deaths: three in the donanemab group and one in the placebo group, but all the fatalities were considered a result of the treatment they received.
The trial recruited participants aged 60 to 85 with early symptomatic Alzheimer's, either mild cognitive impairment or Alzheimer's disease with mild dementia.
The news comes after the first Alzheimer's drug to be approved was pulled from the market in January.
The FDA awarded accelerated approval to Aduhelm in June 2021, a decision that was contentious at the time because the agency overruled its own independent advisors, who found there was insufficient evidence of benefit.
Biogen, which co-developed Aduhelm with Eisai, said it was discontinuing Aduhelm to focus its efforts of Leqembi.
Alzheimer's is the most common form of dementia. More than one in nine people over 65 develop the condition, which worsens over time, robbing them of their memories and independence, according to the US Alzheimer's Association.
O.Schlaepfer--VB




