-
 Pollock shines as England eventually overpower Australia
Pollock shines as England eventually overpower Australia
-
Villarreal crush Rayo to move second, Atletico beat Sevilla

-
 Sinner crushes Zverev to reach Paris Masters final, brink of No. 1
Sinner crushes Zverev to reach Paris Masters final, brink of No. 1
-
Pollock shines as England beat Australia in Autumn opener

-
 Ukraine sends special forces to embattled eastern city
Ukraine sends special forces to embattled eastern city
-
Arsenal cruise against Burnley as Man Utd held

-
 Pollock shines as England beat Australia 25-7 in Autumn Nations Series
Pollock shines as England beat Australia 25-7 in Autumn Nations Series
-
Gyokeres on target as leaders Arsenal beat Burnley

-
 Woman charged over Louvre heist tears up in court
Woman charged over Louvre heist tears up in court
-
Diomande dazzles as Leipzig go two points behind Bayern

-
 Auger-Aliassime downs Bublik to reach Paris Masters final
Auger-Aliassime downs Bublik to reach Paris Masters final
-
Villarreal crush Rayo to move second in La Liga

-
 Female suspect, 38, charged in Louvre heist: AFP
Female suspect, 38, charged in Louvre heist: AFP
-
US not sending any high-level officials to COP30

-
 India captain Kaur sees World Cup final as possible turning point
India captain Kaur sees World Cup final as possible turning point
-
'Not out of the woods': What now for Britain's ex-prince Andrew?

-
 Tens of thousands of Serbians mark first anniversary of deadly train station collapse
Tens of thousands of Serbians mark first anniversary of deadly train station collapse
-
Tanzania president wins 98% in election as opposition says hundreds killed

-
 Vieira 'no longer' manager of troubled Genoa: club
Vieira 'no longer' manager of troubled Genoa: club
-
Tanzania president wins 98% of votes after violence-marred polls

-
 South Korea hosts Xi as Chinese leader rekindles fraught ties
South Korea hosts Xi as Chinese leader rekindles fraught ties
-
England's batting exposed as New Zealand seal ODI series sweep

-
 Funk legend turned painter George Clinton opens show in Paris
Funk legend turned painter George Clinton opens show in Paris
-
Traditional mass wedding held in Nigeria to ensure prosperity

-
 Canada PM says Xi talks 'turning point', apologises to Trump
Canada PM says Xi talks 'turning point', apologises to Trump
-
Iranian tech prodigies battle it out with robots

-
 Maldives begins 'generational ban' on smoking
Maldives begins 'generational ban' on smoking
-
Explorers seek ancient Antarctica ice in climate change study

-
 India's Iyer discharged from hospital after lacerated spleen
India's Iyer discharged from hospital after lacerated spleen
-
Serbia marks first anniversary of deadly train station collapse

-
 Latin America weathered Trump tariffs better than feared: regional bank chief
Latin America weathered Trump tariffs better than feared: regional bank chief
-
Bangladesh dockers strike over foreign takeover of key port

-
 Tanzania president wins election landslide after deadly protests
Tanzania president wins election landslide after deadly protests
-
Dodgers, Blue Jays gear up for winner-take-all World Series game seven

-
 Taiwan's new opposition leader against defence spending hike
Taiwan's new opposition leader against defence spending hike
-
Dodgers hold off Blue Jays 3-1 to force World Series game seven

-
 Crowns, beauty, fried chicken: Korean culture meets diplomacy at APEC
Crowns, beauty, fried chicken: Korean culture meets diplomacy at APEC
-
Panama wins canal expansion arbitration against Spanish company

-
 Myanmar fireworks festival goers shun politics for tradition
Myanmar fireworks festival goers shun politics for tradition
-
China to exempt some Nexperia orders from export ban

-
 Sixers suffer first loss as NBA Cup begins
Sixers suffer first loss as NBA Cup begins
-
China's Xi to meet South Korean leader, capping APEC summit

-
 Japan's Chiba leads after Skate Canada short program
Japan's Chiba leads after Skate Canada short program
-
Finland's crackdown on undocumented migrants sparks fear

-
 Climbers test limits at Yosemite, short-staffed by US shutdown
Climbers test limits at Yosemite, short-staffed by US shutdown
-
Gstaad gives O'Brien record 21st Breeders' Cup win

-
 After the tears, anger on Rio's blood-stained streets
After the tears, anger on Rio's blood-stained streets
-
Sinner boosts number one bid in Paris, to face Zverev in semis

-
 Springer back in Toronto lineup as Blue Jays try to close out Dodgers
Springer back in Toronto lineup as Blue Jays try to close out Dodgers
-
Nationals make Butera MLB's youngest manager since 1972


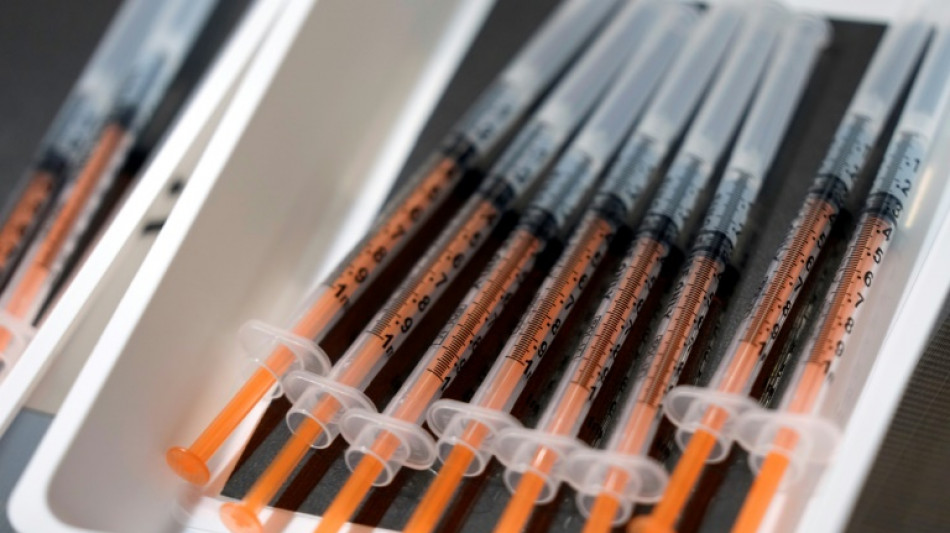
Moderna seeks US authorization for Covid vaccine in children under 6: statement
US biotech firm Moderna said Thursday it had submitted a request for an emergency use authorization in the United States for its Covid vaccine for children aged six months to under six years.
Children under six are the only age group that has yet to gain access to a Covid-19 vaccine in the United States and in most countries.
"We believe (this vaccine) will be able to safely protect these children against SARS-CoV-2, which is so important in our continued fight against Covid-19 and will be especially welcomed by parents and caregivers," the company's CEO Stephane Bancel said in a statement.
In March, the company announced results from a trial that showed the two-shot regimen was found to be safe and produced a strong immune response.
Specifically, two doses of 25 micrograms given to babies, toddlers and preschoolers generated similar levels of antibodies as two doses of 100 micrograms given to young people aged 18-25, indicating there would be similar levels of protection against serious cases of the virus.
The trial included 4,200 children aged two to six years and 2,500 babies aged six months to two years.
Side effects were generally mild and consistent with those seen in older age groups
The company did, however, find relatively low efficacy against infection, with its trial taking place during the Omicron variant wave.
The current generation of vaccines were designed against the original strain of the virus.
Vaccine efficacy in children six months up to age two was 51 percent, and efficacy was 37 percent in the two to five years age group, when limiting the analysis to only cases confirmed positive on a positive PCR test.
Moderna said these were similar to vaccine efficacy estimates in adults during Omicron, and it is also currently studying booster doses for all pediatric cohorts.
The lower efficacy for two doses has the potential to present a stumbling block to authorization.
Back in February, the Food and Drug Administration (FDA) postponed a meeting of a panel to consider the Pfizer-BioNTech Covid vaccine for children younger than five, saying it wanted to see data on how three doses performed before considering the matter.
The companies said at the time they expected that data to be ready in April, but haven't provided an update since then.
Scientists evaluating a vaccine for infants must closely consider the risk-benefit balance.
Even when they are unvaccinated, children under five are at very low risk for severe disease. There have been only 476 deaths in the United States this age group since the start of the pandemic, according to official data.
Among all US children, there have also been almost 8,000 cases of MIS-C, a post-viral inflammatory condition, that caused 66 deaths.
R.Adler--BTB



