-
 Liverpool down Real Madrid in Champions League, Bayern edge PSG
Liverpool down Real Madrid in Champions League, Bayern edge PSG
-
Van Dijk tells Liverpool to keep calm and follow Arsenal's lead

-
 PSG left to sweat on injuries to Dembele and Hakimi
PSG left to sweat on injuries to Dembele and Hakimi
-
Reddit, Kick to be included in Australia's social media ban

-
 Ex-Zimbabwe cricket captain Williams treated for 'drug addiction'
Ex-Zimbabwe cricket captain Williams treated for 'drug addiction'
-
Padres ace Darvish to miss 2026 MLB season after surgery

-
 Diaz hero and villain as Bayern beat PSG in Champions League showdown
Diaz hero and villain as Bayern beat PSG in Champions League showdown
-
Liverpool master Real Madrid on Alexander-Arnold's return

-
 Van de Ven back in favour as stunning strike fuels Spurs rout
Van de Ven back in favour as stunning strike fuels Spurs rout
-
Juve held by Sporting Lisbon in stalling Champions League campaign

-
 New lawsuit alleges Spotify allows streaming fraud
New lawsuit alleges Spotify allows streaming fraud
-
Stocks mostly drop as tech rally fades

-
 LIV Golf switching to 72-hole format in 2026: official
LIV Golf switching to 72-hole format in 2026: official
-
Manchester City have become 'more beatable', says Dortmund's Gross

-
 Merino brace sends Arsenal past Slavia in Champions League
Merino brace sends Arsenal past Slavia in Champions League
-
Djokovic makes winning return in Athens

-
 Napoli and Eintracht Frankfurt in Champions League stalemate
Napoli and Eintracht Frankfurt in Champions League stalemate
-
Arsenal's Dowman becomes youngest-ever Champions League player

-
 Cheney shaped US like no other VP. Until he didn't.
Cheney shaped US like no other VP. Until he didn't.
-
Pakistan edge South Africa in tense ODI finish in Faisalabad

-
 Brazil's Lula urges less talk, more action at COP30 climate meet
Brazil's Lula urges less talk, more action at COP30 climate meet
-
Barca's Lewandowski says his season starting now after injury struggles

-
 Burn urges Newcastle to show their ugly side in Bilbao clash
Burn urges Newcastle to show their ugly side in Bilbao clash
-
French pair released after 3-year Iran jail ordeal

-
 Getty Images largely loses lawsuit against UK AI firm
Getty Images largely loses lawsuit against UK AI firm
-
Cement maker Lafarge on trial in France over jihadist funding

-
 Sculpture of Trump strapped to a cross displayed in Switzerland
Sculpture of Trump strapped to a cross displayed in Switzerland
-
Pakistan's Rauf and Indian skipper Yadav punished over Asia Cup behaviour

-
 Libbok welcomes 'healthy' Springboks fly-half competition
Libbok welcomes 'healthy' Springboks fly-half competition
-
Reeling from earthquakes, Afghans fear coming winter

-
 Ronaldo reveals emotional retirement will come 'soon'
Ronaldo reveals emotional retirement will come 'soon'
-
Munich's surfers stunned after famed river wave vanishes

-
 Iran commemorates storming of US embassy with missile replicas, fake coffins
Iran commemorates storming of US embassy with missile replicas, fake coffins
-
Gauff sweeps Paolini aside to revitalise WTA Finals defence

-
 Shein vows to cooperate with France in probe over childlike sex dolls
Shein vows to cooperate with France in probe over childlike sex dolls
-
Young leftist Mamdani on track to win NY vote, shaking up US politics

-
 US government shutdown ties record for longest in history
US government shutdown ties record for longest in history
-
King Tut's collection displayed for first time at Egypt's grand museum

-
 Typhoon flooding kills over 40, strands thousands in central Philippines
Typhoon flooding kills over 40, strands thousands in central Philippines
-
Trent mural defaced ahead of Liverpool return

-
 Sabalenka to face Kyrgios in 'Battle of Sexes' on December 28
Sabalenka to face Kyrgios in 'Battle of Sexes' on December 28
-
Experts call for global panel to tackle 'inequality crisis'

-
 Backed by Brussels, Zelensky urges Orban to drop veto on EU bid
Backed by Brussels, Zelensky urges Orban to drop veto on EU bid
-
After ECHR ruling, Turkey opposition urges pro-Kurd leader's release

-
 UK far-right activist Robinson cleared of terror offence over phone access
UK far-right activist Robinson cleared of terror offence over phone access
-
World on track to dangerous warming as emissions hit record high: UN

-
 Nvidia, Deutsche Telekom unveil 1-bn-euro AI industrial hub
Nvidia, Deutsche Telekom unveil 1-bn-euro AI industrial hub
-
Which record? Haaland warns he can get even better

-
 Football star David Beckham hails knighthood as 'proudest moment'
Football star David Beckham hails knighthood as 'proudest moment'
-
Laurent Mauvignier wins France's top literary award for family saga


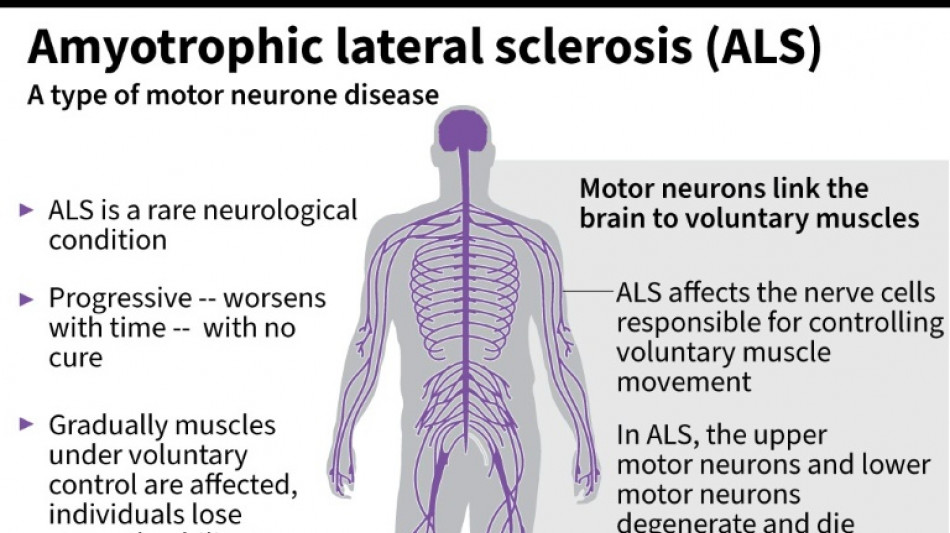
US company withdraws ALS drug after it fails in trial
Amylyx Pharmaceuticals announced Thursday it was withdrawing its approved treatment against the deadly neurodegenerative disease ALS after clinical data found no evidence the drug worked.
In a statement, the US company said it would discontinue its market authorizations for Relyvrio/Albrioza, using the brand names of the medicine in the US and Canadian markets.
"While this is a difficult moment for the ALS community, we reached this path forward in partnership with the stakeholders who will be impacted and in line with our steadfast commitment to people living with ALS and other neurodegenerative diseases," said the company's co-CEOs Joshua Cohen and Justin Klee in a statement.
The company also said it was reducing its workforce "by approximately 70 percent" as it focused on another experimental drug for use against ALS, and on repurposing Relyvrio for other conditions. It added it would continue to make Relyvrio available for patients who wish to keep using the treatment, through a "free drug program."
The news follows data from a clinical trial of 664 ALS patients announced in March, which found no significant differences in outcomes between those on the treatment group and those who received a placebo.
It was a big blow for patients with amyotrophic lateral sclerosis, sometimes called Lou Gehrig's disease after the famous baseball player, which devastates nerve cells in the brain and spinal cord.
ALS affects about two people per 100,000 every year, causing progressive loss of motor and cognitive function. Most patients die within five years of their diagnosis.
Relyvrio's approval by the US Food and Drug Administration in 2022 was controversial and based on the results of a single trial that involved just 137 participants.
The FDA itself noted there was "residual uncertainty about the evidence of effectiveness" -- but "given the serious and life-threatening nature of ALS and the substantial unmet need, this level of uncertainty is acceptable in this instance and consideration of these results in the context of regulatory flexibility is appropriate."
- Patient groups backed approval -
Advocacy groups also mounted a major campaign sending a petition to the FDA with tens of thousands of signatures urging approval. Once it became available, Amylyx reportedly announced an eye-watering list price of $158,000 per year in the US, drawing criticism.
Patient groups in Europe watched with desperation at the bureaucratic delays.
When the European Union drug watchdog later announced it was rejecting Relyvrio, the decision was slammed as "an affront" by angry French patients, who say they "don't have time to wait." France later relented, offering conditional approval in November.
"We commend Amylyx for pulling Relyvrio off the market, while still ensuring that people living with ALS can access the drug if they believe it is helping them," said the US-based ALS association, which had lobbied for the drug's approval and funded its research.
"Safe and potentially effective treatments can be made accessible rapidly until further research can confirm their efficacy," it added.
For now, there remain only a handful of treatments available.
Riluzole, FDA approved in 1995, prolongs life approximately three months. Edaravone, FDA approved in 2017, has been found to slow disease progression and improve survival.
And in 2023, the regulatory body approved tofersen, a gene therapy treatment that targets those ALS cases that are caused by mutations in the SOD1 gene.
C.Stoecklin--VB




