-
 Spain PM vows 'climate pact' on visit to fire-hit region
Spain PM vows 'climate pact' on visit to fire-hit region
-
Serbia's president vows 'strong response' after days of unrest

-
 Brazilian goalkeeper Fabio equals Shilton record for most games played
Brazilian goalkeeper Fabio equals Shilton record for most games played
-
Warholm in confident swagger towards Tokyo worlds

-
 Air Canada to resume flights after govt directive ends strike
Air Canada to resume flights after govt directive ends strike
-
Israelis rally nationwide calling for end to Gaza war, hostage deal

-
 European leaders to join Zelensky for Ukraine talks with Trump
European leaders to join Zelensky for Ukraine talks with Trump
-
Downgraded Hurricane Erin lashes Caribbean with rain

-
 Protests held across Israel calling for end to Gaza war, hostage deal
Protests held across Israel calling for end to Gaza war, hostage deal
-
Hopes for survivors wane as landslides, flooding bury Pakistan villages

-
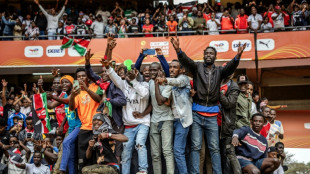 After deadly protests, Kenya's Ruto seeks football distraction
After deadly protests, Kenya's Ruto seeks football distraction
-
Bolivian right eyes return in elections marked by economic crisis

-
 Drought, dams and diplomacy: Afghanistan's water crisis goes regional
Drought, dams and diplomacy: Afghanistan's water crisis goes regional
-
'Pickypockets!' vigilante pairs with social media on London streets

-
 From drought to floods, water extremes drive displacement in Afghanistan
From drought to floods, water extremes drive displacement in Afghanistan
-
Air Canada flights grounded as government intervenes in strike

-
 Women bear brunt of Afghanistan's water scarcity
Women bear brunt of Afghanistan's water scarcity
-
Reserve Messi scores in Miami win while Son gets first MLS win

-
 Japan's Iwai grabs lead at LPGA Portland Classic
Japan's Iwai grabs lead at LPGA Portland Classic
-
Trump gives Putin 'peace letter' from wife Melania

-
 Alcaraz to face defending champ Sinner in Cincinnati ATP final
Alcaraz to face defending champ Sinner in Cincinnati ATP final
-
Former pro-democracy Hong Kong lawmaker granted asylum in Australia

-
 All Blacks beat Argentina 41-24 to reclaim top world rank
All Blacks beat Argentina 41-24 to reclaim top world rank
-
Monster birdie gives heckled MacIntyre four-stroke BMW lead

-
 Coffee-lover Atmane felt the buzz from Cincinnati breakthrough
Coffee-lover Atmane felt the buzz from Cincinnati breakthrough
-
Coffe-lover Atmane felt the buzz from Cincinnati breakthrough

-
 Monster birdie gives MacIntyre four-stroke BMW lead
Monster birdie gives MacIntyre four-stroke BMW lead
-
Hurricane Erin intensifies offshore, lashes Caribbean with rain

-
 Kane lauds Diaz's 'perfect start' at Bayern
Kane lauds Diaz's 'perfect start' at Bayern
-
Clashes erupt in several Serbian cities in fifth night of unrest

-
 US suspends visas for Gazans after far-right influencer posts
US suspends visas for Gazans after far-right influencer posts
-
Defending champ Sinner subdues Atmane to reach Cincinnati ATP final

-
 Nigeria arrests leaders of terror group accused of 2022 jailbreak
Nigeria arrests leaders of terror group accused of 2022 jailbreak
-
Kane and Diaz strike as Bayern beat Stuttgart in German Super Cup

-
 Australia coach Schmidt hails 'great bunch of young men'
Australia coach Schmidt hails 'great bunch of young men'
-
Brentford splash club-record fee on Ouattara

-
 Barcelona open Liga title defence strolling past nine-man Mallorca
Barcelona open Liga title defence strolling past nine-man Mallorca
-
Pogba watches as Monaco start Ligue 1 season with a win

-
 Canada moves to halt strike as hundreds of flights grounded
Canada moves to halt strike as hundreds of flights grounded
-
Forest seal swoop for Ipswich's Hutchinson

-
 Haaland fires Man City to opening win at Wolves
Haaland fires Man City to opening win at Wolves
-
Brazil's Bolsonaro leaves house arrest for medical exams

-
 Mikautadze gets Lyon off to winning start in Ligue 1 at Lens
Mikautadze gets Lyon off to winning start in Ligue 1 at Lens
-
Fires keep burning in western Spain as army is deployed

-
 Captain Wilson scores twice as Australia stun South Africa
Captain Wilson scores twice as Australia stun South Africa
-
Thompson eclipses Lyles and Hodgkinson makes stellar comeback

-
 Spurs get Frank off to flier, Sunderland win on Premier League return
Spurs get Frank off to flier, Sunderland win on Premier League return
-
Europeans try to stay on the board after Ukraine summit

-
 Richarlison stars as Spurs boss Frank seals first win
Richarlison stars as Spurs boss Frank seals first win
-
Hurricane Erin intensifies to 'catastrophic' category 5 storm in Caribbean


US becomes first country to approve RSV vaccine
The United States on Wednesday approved the world's first vaccine for the Respiratory Syncytial Virus (RSV), the culmination of a decades-long hunt to protect vulnerable people from the common illness.
Drugmaker GSK's Arexvy was green-lighted for adults aged 60 and older, with similar shots from other makers including Pfizer and Moderna expected to follow soon.
"Today's approval of the first RSV vaccine is an important public health achievement to prevent a disease which can be life-threatening," said senior US Food and Drug Administration (FDA) official Peter Marks in a statement.
The decision "marks a turning point in our effort to reduce the significant burden of RSV," added Tony Wood, GSK's chief scientific officer.
RSV is a common virus that normally causes mild, cold-like symptoms, but can be serious for infants and the elderly, as well as those with weak immune systems and underlying conditions.
In severe cases it can cause pneumonia and bronchiolitis, an inflammation of the small airways deep inside the lungs.
According to the US Centers for Disease Control and Prevention, RSV leads to approximately 60,000 to 120,000 hospitalizations and 6,000 to 10,000 deaths among adults 65 years of age and older.
Awareness of the disease has increased in recent years, in part because of the strain it has placed on hospital systems over the last two winters.
Rates of RSV and flu fell during Covid-19 lockdowns, but surged when restrictions were lifted, with young children hit hard.
Pharmaceutical companies have been chasing an RSV vaccine for years. Given recent successful breakthroughs in the sector, analysts predict the market could be worth over $10 billion in the next decade, according to reports.
- More vaccines on way -
GSK's vaccine contains a "subunit" or part of the virus to help train the immune system should it encounter the real thing.
It was approved based on a study of 25,000 people aged 60 and older that showed a single dose was 83 percent effective against disease caused by RSV, and more than 94 percent effective against severe disease.
Researchers will continue to follow volunteers in the study to assess the duration of protection as well as the safety and efficacy of more doses.
The most common side effects included injection site pain, fatigue, muscle pain, headaches and joint stiffness.
An irregular heartbeat was a less common side effect, occurring in 10 participants who received Arexvy and four participants who received placebo.
Safety issues were also found in two other studies of the drug involving approximately 2,500 people aged 60 and up. In one of these studies, two volunteers developed a rare type of inflammation that affects the brain and spinal cord, and one of them died.
In the other study, one participant developed Guillain-Barre syndrome, in which the immune system damages nerve cells, causing muscle weakness and sometimes paralysis.
GSK's Arexvy was recommended for approval last week by the European Union's drug watchdog, the European Medicines Agency, whose positive opinions are normally formally followed by approval from the European Commission.
Pfizer has said that it expects a decision from the FDA in May for its own RSV vaccine, also for those over 60 years old.
In January, Moderna said it hopes its RSV vaccine will be approved and available in time for the Northern Hemisphere's winter later this year.
Several other companies are also developing RSV vaccines.
Last year, the EU approved a preventative antibody treatment against RSV, developed by British-Swedish pharmaceutical firm AstraZeneca and France's Sanofi, which confers temporary protection.
H.Seidel--BTB