
-
 Syria army enters Al-Hol camp holding relatives of jihadists: AFP
Syria army enters Al-Hol camp holding relatives of jihadists: AFP
-
Brook apologises, admits nightclub fracas 'not the right thing to do'

-
 NATO chief says 'thoughtful diplomacy' only way to deal with Greenland crisis
NATO chief says 'thoughtful diplomacy' only way to deal with Greenland crisis
-
Widow of Iran's last shah says 'no turning back' after protests

-
 Waugh targets cricket's 'last great frontier' with European T20 venture
Waugh targets cricket's 'last great frontier' with European T20 venture
-
Burberry sales rise as China demand improves

-
 Botswana warns diamond oversupply to hit growth
Botswana warns diamond oversupply to hit growth
-
Spaniard condemns 'ignorant drunks' after Melbourne confrontation

-
 Philippines to end short-lived ban on Musk's Grok chatbot
Philippines to end short-lived ban on Musk's Grok chatbot
-
Police smash European synthetic drug ring in 'largest-ever' op

-
 Japan to restart world's biggest nuclear plant Wednesday
Japan to restart world's biggest nuclear plant Wednesday
-
South Korean ex-PM Han gets 23 years jail for martial law role

-
 Alcaraz, Sabalenka, Gauff surge into Australian Open third round
Alcaraz, Sabalenka, Gauff surge into Australian Open third round
-
Over 1,400 Indonesians left Cambodian scam groups in five days: embassy

-
 Raducanu to 're-evaluate' after flat Australian Open exit
Raducanu to 're-evaluate' after flat Australian Open exit
-
Doncic triple-double leads Lakers comeback over Nuggets, Rockets down Spurs

-
 Bangladesh will not back down to 'coercion' in India T20 World Cup row
Bangladesh will not back down to 'coercion' in India T20 World Cup row
-
Alcaraz comes good after shaky start to make Australian Open third round

-
 Trump departs for Davos forum again after switching to new plane: AFP
Trump departs for Davos forum again after switching to new plane: AFP
-
Impressive Gauff storms into Australian Open third round

-
 Dazzling Chinese AI debuts mask growing pains
Dazzling Chinese AI debuts mask growing pains
-
Medvedev battles into Melbourne third round after early scare

-
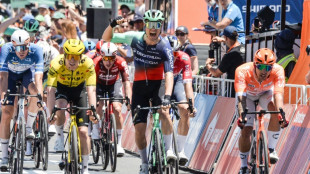 Denmark's Andresen upstages sprint stars to take Tour Down Under opener
Denmark's Andresen upstages sprint stars to take Tour Down Under opener
-
Turkey's Sonmez soaks in acclaim on historic Melbourne run

-
 Sheppard leads Rockets to sink Spurs in Texas derby
Sheppard leads Rockets to sink Spurs in Texas derby
-
Sabalenka shuts down political talk after Ukrainian's ban call

-
 Trump's plane returns to air base after 'minor' electrical issue: White House
Trump's plane returns to air base after 'minor' electrical issue: White House
-
Barcelona train crash kills 1 in Spain's second deadly rail accident in days

-
 North produces enough nuclear material a year for 10-20 weapons: S. Korea president
North produces enough nuclear material a year for 10-20 weapons: S. Korea president
-
Japan ex-PM Abe's alleged killer faces verdict

-
 Climate change fuels disasters, but deaths don't add up
Climate change fuels disasters, but deaths don't add up
-
Stocks stable after tariff-fuelled selloff but uncertainty boosts gold
-
 What growth?: Taiwan's traditional manufacturers miss out on export boom
What growth?: Taiwan's traditional manufacturers miss out on export boom
-
'Super-happy' Sabalenka shines as Alcaraz gets set at Australian Open

-
 With monitors and lawsuits, Pakistanis fight for clean air
With monitors and lawsuits, Pakistanis fight for clean air
-
Sabalenka sets up potential Raducanu showdown at Australian Open

-
 Chile president picks Pinochet lawyers as ministers of human rights, defense
Chile president picks Pinochet lawyers as ministers of human rights, defense
-
Osaka says 'I'm a little strange' after Melbourne fashion statement

-
 UN report declares global state of 'water bankruptcy'
UN report declares global state of 'water bankruptcy'
-
Trump heads for Davos maelstrom over Greenland

-
 Ukraine's Oliynykova wants Russian, Belarusian players banned from tennis
Ukraine's Oliynykova wants Russian, Belarusian players banned from tennis
-
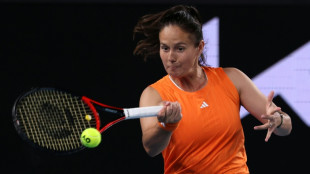
Kasatkina cannot wait to be back after outpouring of Melbourne support
-
 Chile blaze victims plead for help from razed neighborhoods
Chile blaze victims plead for help from razed neighborhoods
-
Russian minister visits Cuba as Trump ramps up pressure on Havana

-
 World order in 'midst of a rupture': Canada PM Carney tells Davos
World order in 'midst of a rupture': Canada PM Carney tells Davos
-
Senegal's 'historic' AFCON champs honoured with parade, presidential praise

-
 Audi unveil new car for 2026 Formula One season
Audi unveil new car for 2026 Formula One season
-
Man City humiliated, holders PSG stumble, Arsenal remain perfect

-
 Vinicius, Real Madrid need 'love' not whistles: Bellingham
Vinicius, Real Madrid need 'love' not whistles: Bellingham
-
Late Suarez winner stops Champions League holders PSG in Lisbon


GEN and Sulfateq BV Announce Positive Phase 1 Trial Data on Investigational Drug SUL-238 for Alzheimer’s and Other Neurodegenerative Diseases
Phase 1 results demonstrate that SUL-238, a first-in-class, orally administered, mitochondria-directed drug candidate, is safe and well-tolerated in healthy elderly volunteers, showing a favourable pharmacokinetic profile and high brain penetration. These findings support the advancement of SUL-238 into further clinical development for Alzheimer's and other neurodegenerative diseases.
ANKARA, TR / ACCESS Newswire / July 28, 2025 / GEN Pharmaceuticals (GENIL.IS), Türkiye's leading specialty pharmaceutical company, announced positive results from its Phase 1 clinical trial evaluating the safety, tolerability, and pharmacokinetics (PK) of first-in-class and novel orally administered mitochondria-directed drug candidate SUL-238 in healthy elderly volunteers. The findings were presented at the Alzheimer's Association International Conference 2025 (AAIC®) in Toronto.
This single oral ascending dose (SAD) Phase 1, first-in-human, randomized, double-blind, placebo-controlled study was conducted in three parts, involving a total of 53 healthy elderly adults. Part 1 included 6 cohorts (50, 100, 250, 500, 1000, and 2000 mg orally, n=23). In Part 2, the PK of a single 1000 mg oral dose was investigated in 10 healthy elderly adults. In Part 2B, the food effect was assessed using a randomized, single oral 2000 mg dose, two-treatment, two-period, crossover design (n=20).
The trial results showed that single oral doses of 50-2000 mg of SUL-238 were safe and well-tolerated, while demonstrating a favourable PK profile and high cerebrospinal fluid (CSF) penetration. These findings make SUL-238 a promising candidate for further clinical development in neurodegenerative diseases, including Alzheimer's disease.
No adverse effects (AEs) limited dose escalation, AE rates were comparable between SUL-238 and placebo, and all AEs were mild or moderate. The mean terminal elimination half-life was 0.86-3.80 hours, and the time to maximum plasma concentration was 0.50-1.39 hours. Under fed conditions, maximum plasma concentration (Cmax) and area under the plasma concentration-time curve (AUC0-∞) decreased by 50% and 60%, respectively. CSF-to-plasma percentages at 2 and 8 hours post-dose were 21.1% (±6.6%) and 74.2% (±46.0%).
Abidin Gülmüş, Chairman of GEN, stated:
"We are very encouraged by these promising first-in-human results, marking an important step forward in our mission to address the underlying biology of Alzheimer's disease."
Nadir Ulu, MD, PhD, Vice President of R&D at GEN, added:
"With its excellent safety and PK profile in this Phase 1 trial, combined with robust preclinical data, SUL-238 represents a strong candidate for further clinical development to meet the critical unmet needs in neurodegenerative diseases, including Alzheimer's disease."
About SUL-238
SUL-238 is a novel, first-in-class, hibernation-derived small molecule that targets mitochondria, the cell's "powerhouse." It supports mitochondrial bioenergetics via complex I/IV activation and has improved mitochondrial function in rodent models of neurodegenerative, cardiovascular, and renal diseases, as well as aging. SUL-238 crosses the blood-brain barrier and has undergone extensive safety evaluation in preclinical and Phase 1 studies. GEN licenses SUL-238 from Sulfateq BV for neurodegenerative disease applications.
About GEN:
Founded in 1998, GEN is Türkiye's leading specialty pharmaceutical company, focused on developing innovative therapies across multiple therapeutic areas. GEN manufactures high-quality, competitive products at its GMP-certified facility and pursues original drug development through two dedicated R&D centers and investments.
About Sulfateq:
Sulfateq B.V. is an early-stage Dutch biotech company that fosters strategic collaborations with academic and industrial research centers to accelerate the development of innovative new medicines. It has developed a novel class of small molecules, the SUL-compounds, that maintain mitochondrial health.
For more information:
www.genilac.com.tr
www.sulfateqbv.com
Contact Information
Bulutay Güneş
Sr. Head of Corporate Brand
[email protected]
Ali Ketencioğlu
Investor Relations Manager
[email protected]
Kees van der Graaf
Sulfateq CEO
[email protected]
SOURCE: GEN İlaç ve Sağlık Ürünleri A.Ş.
View the original press release on ACCESS Newswire
R.Kloeti--VB
